Research:
phase transitions and the pluripotent state
Mouse embryonic stem cells (ESC) are derived from the pre-implantation embryo. In 2007, two groups described a new type of mouse stem cell derived from the post-implantation embryo, called the epiblast stem cell (EpiSC) (Tesar et al., Nature 2007). Although EpiSC can form most tissues, they differ from ESC in their morphological appearance, culture requirements, and gene expression profile. Interestingly, human ESC are similar in their appearance and culture requirements to EpiSC.
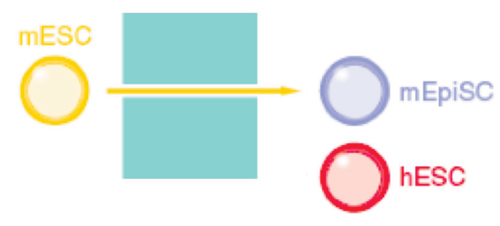
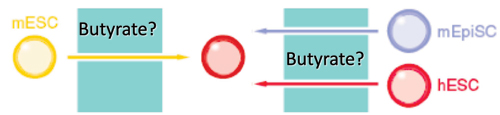
Our collaborator, Carol Ware, discovered that a low, narrow concentration of the histone deacetylase Inhibitor, sodium butyrate, can support the self renewal of ESC from humans, mice, and non-human primates (Ware et al., Cell Stem Cell 2009). Butyrate appears to induce a change in stem cell state. Gene expression profiling suggests that human ESC pulled backward to an earlier developmental stage, whereas mouse ESC are pushed forward.
erythropoietin and cancer
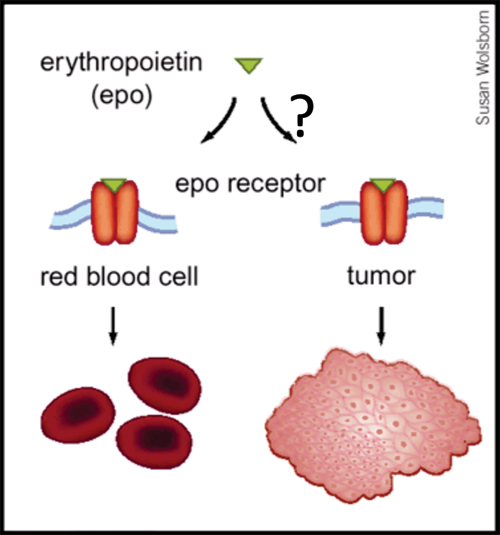
Up to 40% of patients with cancer are anemic at the time of diagnosis, and a large body of evidence indicates that anemia is a key predictor of survival, independent of disease severity. The mechanism whereby anemia impairs outcome in patients with cancer is not well understood, but has been attributed in part to tumor hypoxia, which can select for tumor cells resistant to chemotherapy and radiation therapy. This rationale helped to lay the groundwork for using Epo for the treatment of anemia in patients with cancer. Recombinant Epo first received FDA approval for the treatment of cancer-related anemia in 1993, and subsequently grew to become the most commercially successful drug in all of oncology. However, the results of Phase III clinical trials now indicate that Epo can reduce survival rates and promote tumor progression in cancers of the head and neck, breast and in most common forms of lung cancer. Although some of these trials are not yet published, and several of the published trials have been criticized for design limitations, the FDA issued a "black box" warning for Epo in March 2007. How Epo stimulates tumor progression is uncertain, but may reflect off-target effects (figure, below). EpoR transcripts have been detected in multiple primary cancers including tumors of the breast, head and neck, and non-small cell lung. Some reports have documented Epo-induced proliferation, invasion, migration, and protection from chemo- and radio-therapy in various cancer cell lines. Moreover, blocking endogenous Epo can inhibit breast cancer growth and tumor vascularization in a rat model, and ovarian and uterine cancers in mice. Finally, EpoR expression and function in endothelial cells is well-documented, and Epo might stimulate tumor progression by promoting tumor angiogenesis.
Is Erythropoietin Induced Tumor Progression An Off-Target Effect?
One of the phase III trials suggesting that Epo might promote cancer progression, was ENHANCE, a trial of 351 patients with head and neck cancer (Henke et al., Lancet 2003). ENHANCE demonstrated a shorter locoregional progression free survival (LPFS) in head and neck cancer patients randomized to Epo rather than placebo during radiotherapy. ENHANCE incorporated patients who underwent complete, partial or no resection of tumor prior to radiotherapy. Adverse effects of Epo were confined to patients with residual tumor at the time of radiotherapy. We developed an assay to measure mRNA levels of genes implicated (EpoR, Jak2, Csf2rb, Hsp70) or not implicated (Krt5, Cd44) in Epo signaling in formalin-fixed paraffin-embedded (FFPE) tumors, and tested 136 tumors from ENHANCE. We compared LPFS between patients with tumors expressing above- versus below-median levels of the aforementioned mRNAs using the log rank statistic. Sufficient RNA for EpoR measurements was available in 101 tumors and EpoR varied over a 30-fold range. There was no association between tumor EpoR level and LPFS across all 101 patients. However in patients with unresected tumors (n=28), above-median EpoR mRNA levels were associated with significantly poorer LPFS if randomized to Epo rather than placebo (p=0.02, n=14). A similar association was observed in patients with above-median levels of Jak2 mRNA (p=0.02, n=18) or below-median levels of Hsp70 (p=0.01, n=20). LPFS was not significantly different when comparing Epo-treated patients with above-median mRNA levels to Epo-treated patients with below-median levels. EpoR mRNA levels can be reliably measured in FFPE tumors. These associations merit evaluation in larger numbers of tumors from other Phase III trials.
pharmacologically regulated cell therapy
We are developing a new generation of cell therapies that involves placing cells under the "remote control" of small molecule drugs. Over the past decade we have completed much of the ground-work establishing the feasibility of this approach. We believe that the approaches we are developing today will be commonplace 100 years from now (see the last chapter of "Thomas' Hematopoietic Cell Transplantation," written by Ernie Beutler), but we are most interested in pursuing approaches with (hopefully) nearer-term clinical applications.
Off Target Effects Constrain the Clinical Applicability of Growth Factors. The safety and efficacy of a drug are determined by two forms of specificity: specificity of the drug for its target (usually a protein), and specificity of the drug target in the pathogenesis of the disease being treated. Side effects arise when either type of specificity falls short of physiologically dictated thresholds, phenomena collectively referred to as "off-target" effects. Although off-target effects plague all drug development, their impact on the clinical use of hematopoietic growth factors provide a case-in-point, and a little explored rationale for genetic manipulation.
The major hematopoietic growth factors in clinical use today, erythropoietin (Epo), granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) were all approved by the US Food and Drug Administration (FDA) more than 15 years ago. Since then no growth factors with novel biological activity have gained widespread use, a situation almost entirely attributable to off-target effects. For example, none of the many potential clinical uses for recombinant stem cell factor (SCF) were realized because its receptor (c-kit) is expressed not only in primitive hematopoietic progenitors, but also in mast cells, causing significant allergic reactions in 10 - 20% of patients. Fibroblast growth factors (FGFs) further illustrate the problem. 23 different FGFs bind 7 different receptor isoforms that are variably expressed in all tissues. Most FGFs activate more than one type of FGF receptor, leading to one type of off target effect. Furthermore, even when an FGF specific for a given receptor is used, the receptor is invariably expressed in multiple cell types, producing a second type of off target effect. Thus clinical trials of FGFs have yielded disappointing results. Over the past decade we have been developing a system that has the potential to circumvent off-target effects.
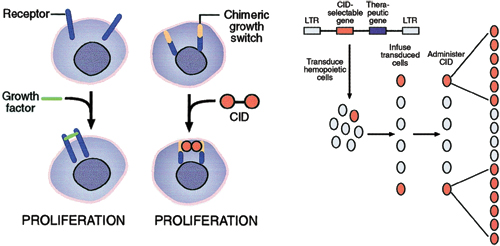
Pharmacologically Regulated Cell Therapy. An important obstacle to cell therapy is the loss of control over cells that have been transplanted. We used previously described technology to develop a way to regulate the proliferation of engineered cells using a growth factor receptor modified to substitute its normal ligand-binding site with the binding site for a drug called a chemical inducer of dimerization (CID). The CID brings together two copies of the artificial receptor, triggering its activation and leading to cell proliferation, thereby mimicking the effect of a growth factor.
Regulated Cell Therapy
genome architecture analysis
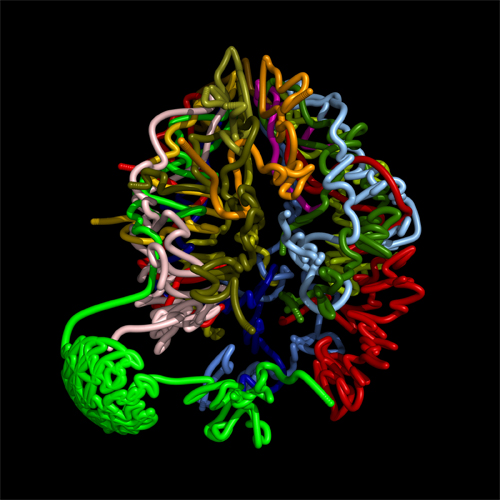
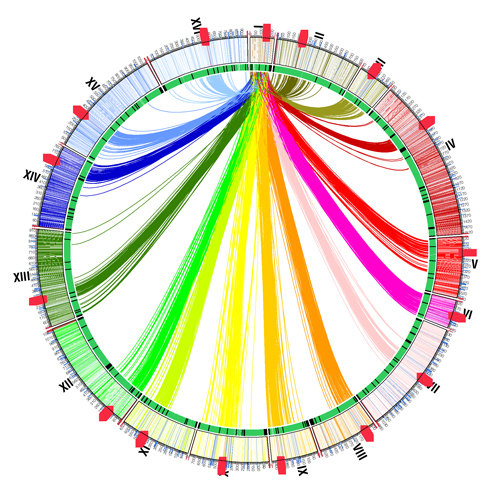
One of the fundamental questions of the post-genome era is: How does genotype (an organism's genetic structure) translate into phenotype (an observable characteristic)? Our genetic information is contained in 23 pairs of chromosomes. The 46 chromosomes are arranged not in straight lines, but in a tangle, like a plate of cooked spaghetti. The result is that a gene's nearest neighbors in three-dimensional space may be on completely different chromosomes. In collaboration with the laboratories of Dr. Bill Noble, Dr. Stan Fields, and Dr. Jay Shendure (all faculty members in the Department of Genome Sciences), Zhijun Duan in our lab developed a method for capturing interactions within and between chromosomes genome-wide. Mirela Andronescu (Noble Lab) used this information to construct a three-dimensional model of the genome of budding yeast (Duan et al., Nature)
The information provides an entirely new epigenetic view of the genome, and will likely provide important insights into cell state, cell identity, cancer, and evolution. We plan to use this new method to characterize the genomic architecture of stem cells, and to understand how this changes during stem cell differentiation.
A Three-Dimensional Model of the Yeast Genome
a smarter war on cancer
Cancers are far more variable on a molecular level than they appear under the microscope. Still, we continue to treat most cancers based on their appearance – an approach nearly as crude as giving a blood transfusion to everyone who complains of fatigue. Today, however, emerging genomic and computational technologies are transforming cancer research. That research, in turn, promises to transform cancer treatment as well.
An "N of 1" approach
The Blau Lab is establishing the infrastructure to treat a small number of highly motivated cancer patients as individual experiments: in scientific parlance, an "N of 1." Vast amounts of data will be analyzed from each patient's tumor to predict which proteins should be targeted in order to destroy the cancer. Each patient would then receive a drug regimen tailored to his or her tumor. In this kind of single-subject approach, there is no control group against which to compare the response of the experimental subject. Instead, each patient would serve as his or her own control, using a technique called serial molecular monitoring. In this technique, the patient would receive a drug designed to block a particular target protein. A biopsy would then be performed to confirm whether the drug had worked and the target had indeed been blocked. In this way, researchers would track the tumor's molecular response to treatment through repeated biopsies (a requirement that may eventually be replaced by sampling blood). One of the most important advantages of serial molecular monitoring is that it would reveal strategies that tumors adopt to evade therapy, possibly uncovering new targets of opportunity.
This patient-centered approach represents a dramatic departure from traditional oncology. Because these novel patient-specific combinations of drugs could have unforeseen side effects, the methodological, regulatory, and ethical framework for cancer research would need to be reconsidered from the ground up. Therapies that appear to be effective would be validated in small trials involving other patients with similar molecular profiles. Unsuccessful therapies could be analyzed to refine our understanding of tumor biology and drug mechanisms. The N of 1 approach may not hit immediate home runs. However, the extensive body of knowledge generated from each patient should, upon aggregation with data from other patients, enable us to tell from a blood sample which patients will respond to particular drugs. In time, we hope to be able to stop tumors by anticipating the escape routes they are likely to take.
Although this approach will be very expensive for early adopters, technology costs are falling at exponential rates (think of Moore's Law for integrated circuits). Eventually, the approach should lead to dramatic reductions in health-care costs. For one thing, the approach allows us to administer these expensive drugs only to patients for whom they are most likely to work. For another, the costs of the approach will go down and – and scalability increase – once expected advances in technology allow us to use simple blood draws rather than repeated tumor biopsies.
Although many significant challenges remain, the primary roadblock to implementing the single-subject approach is financial. Will insurance companies or federal agencies pay to test this new paradigm for cancer treatment? Will patients with difficult-to-treat cancers (and the means to fund their own treatment) be willing to take on this grand experiment – for their own benefit, potentially, and for the benefit of other patients? We believe that it's time to find out.
Selected Publications:
Blau CA, Peterson KR, Drachman JG and Spencer DM: A proliferation switch for genetically modified cells. PNAS USA 94:3076-81, 1997.
Abboud M, Laver J and Blau CA: Granulocytosis causing sickle-cell crisis. Lancet 351:609, 1998.
Jin L, Siritanaraktul N, Emery DW, Richard RE, Kaushansky K, Papayannopoulou Th and Blau CA: Targeted expansion of genetically modified bone marrow cells. PNAS USA 95:8093-7, 1998.
Jin L, Zeng H, Otto KG, Richard RE, Emery DW and Blau CA: In vivo selection using a cell growth switch. Nat. Genet. 26:64-66, 2000.
Zeng H, Masuko M, Jin L, Neff T, Otto KG and Blau CA: Receptor specificity in the self-renewal and differentiation of primary multipotential hemopoietic cells. Blood 98:328-34, 2001.
Neff T and Blau CA: Pharmacologically regulated cell therapy (review). Blood 97:2535-2540, 2001.
Zhao S, Zoller K, Masuko M, Rojnuckarin P, Yang X, Parganas E, Kaushansky K, Ihle JN, Papayannopoulou Th, Willerford DM, Clackson T and Blau CA: JAK2, complemented by a second signal from c-kit or flt-3, triggers extensive self-renewal of primary multipotential hemopoietic cells. EMBO J. 21:2159-67, 2002.
Neff T, Horn PA, Valli VE, Gown AM, Wardwell S, Wood BL, von Kalle C, Schmidt M, Peterson LJ, Morris JC, Richard RE, Clackson T, Kiem HP, Blau CA: Pharmacologically regulated in vivo selection in a large animal. Blood 100:2026-31, 2002.
Richard RE and Blau CA: Selective expansion of genetically modified cord blood. Stem Cells 21:71-78, 2003.
Richard RE, Weinreich M, Chang K-H, Ieremia J, Stevenson MM, Blau CA: Modulating erythrocyte mixed chimerism in a mouse model of pyruvate kinase deficiency. Blood 103:4432-39, 2004.
Zhao S, Weinreich MA, Ihara K, Richard RE, Blau CA: In vivo selection using a JAK2-based cell growth switch. Molecular Therapy 10:456-68, 2004.
Richard RE, De Claro A, Yan J, Chien S, Kiem HP, Clackson T, Andrews R, Blau CA: Heterogeneity among large animal models of in vivo selection. Molecular Therapy 10:730-740, 2004.
Ware CB, Chien S, Blau CA: Slow, controlled-rate freezing improves human embryonic stem cell survival. Biotechniques 38:879-883, 2005.
Gandhi M, Ihara K, Cummings C, Pendergrass T, Blau CA, Drachman JD: Congenital megakaryocytic thrombocytopenia in three siblings: molecular analysis of atypical clinical presentation. Exp. Hematol. 33:1215-21, 2005.
Blau CA, Yan J, Neades R, Navas PA, Peterson KR: Gamma-globin gene expression in CID-dependent multi-potential cells established from beta-YAC transgenic mice. J. Biol. Chem. 280:36642-47, 2005.
Richard RE, Siritanaratkul N, Jonlin E, Skarpidi E, Heimfeld S, Blau CA: Collection of blood stem cells from patients with sickle cell anemia. Blood Cells, Molecules & Diseases 35:384-8, 2005.
Nagasawa Y, Wood B, Wang L, Lintmaier I, Guo W, Papayannopoulou Th, Harkey M, Nourigat C, Blau CA: Anatomical compartments modify the response of human hematopolietic cells to a mitogenic signal. Stem Cells 24:908-17, 2006.
Weinreich MA, Lintmaer I, Wang L, Liggitt D, Harkey M, Blau CA: Growth factor receptors as regulators of hematopoiesis. Blood 108):3713-21, 2006.
Ware CB, Nelson, AM, and Blau CA: A Comparison of NIH-approved human embryonic stem cell lines. Stem Cells 24:2677-84, 2006.
Harkey MA, Kaul R, Jacobs MA, Kurre P, Torok-Storb B, Blau CA: Multi-arm high-throughput LAM-PCR: an optimized approach to clonal analysis. Stem Cells & Dev. 16:381-392, 2007.
Blau CA: Erythropoietin in cancer: Presumption of innocence? Stem Cells 25:2094-7, 2007.
Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song C-Z, Chen X, Brimble SN, Amanda M, Galeano MJ, Uhl EW, Damour KA, Chesnut J, Rao MS, Blau CA* and Robins AJ*: Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 110:4111-9, 2007. *co-corresponding authors.
Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kunzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M. Venous Thromboembolism and Mortality Associated with Recombinant Erythropoietin and Darbepoietin Administration for the Treatment of Cancer-Associated Anemia. JAMA 299: 914-924, 2008.
Duan Z, Andronescu M, Schutz K, McIlwain S, Kim YJ, Lee C, Shendure J, Fields S, Blau CA*, Noble WS*. A three-dimensional model of the yeast genome. Nature. 2010 May 20;465(7296):363-7. Epub 2010 May 2.PMID:
20436457
denotes co-corresponding authors
Miller CA, Blau CA: Using gene transfer to circumvent off-target effects. Gene Therapy, in press.
additional publication listings available via PubMed

